Multi-Biomarker Approach Achieves 61 Percent Sensitivity for Cancer at an Overall Specificity of 98.2 Percent in Robust Case-Control Study Including Nearly 600 Cancer Samples
Data will Inform the Largest Registrational Multi-Cancer Early Detection Study Ever Conducted in the United States to Support Another Leading Test in Exact Sciences' Screening Portfolio
MADISON, Wis., Sept. 10, 2022 /PRNewswire/ -- Exact Sciences Corp. (Nasdaq: EXAS), a leading provider of cancer screening and diagnostic tests, today announced data from a multi-cancer early detection (MCED) biomarker validation study was presented at the European Society for Medical Oncology (ESMO) Congress. The study rigorously assessed the performance of four distinct biomarker classes found in the blood and known to signal the presence of cancer regardless of its location in the body.

"Cancer releases a diverse set of biomarkers into the blood that can be harnessed to detect the devastating disease at earlier stages," said Tom Beer, M.D., F.A.C.P., chief medical officer and vice president, multi-cancer early detection, Exact Sciences. "These data demonstrate in a large, well-designed case-control study that combining different cancer biomarkers improves cancer detection, especially in stages I and II, when treatment may be more effective for patients. This is a major step forward in our mission to detect cancer earlier before signs and symptoms appear."
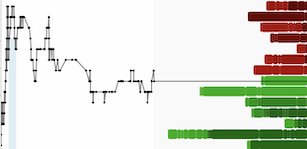
Four biomarker classes, discovered through years of collaboration with Johns Hopkins and Mayo Clinic and analyzed together for the first time in this study, demonstrated the ability to detect cancer signal from 15 organ sites with a mean sensitivity of 61% and mean specificity of 98.2%. The multi-biomarker approach, including aneuploidy, proteins, and DNA methylation and mutations, provided encouraging cancer detection in stages I and II, with a combined sensitivity of 38.7%.
Sensitivities by Cancer Stage for Four-Biomarker Class Set
Stage | Sensitivity |
I | 31.4 % |
II | 45.8 % |
I+II | 38.7 % |
III | 68.3 % |
I+II+III | 49.8 % |
IV | 87.1 % |
The retrospective, case-control study included cancers from 11 organ sites with no recommended screening option today and 15 organ sites and tissue in total, including breast, bladder, colon, esophageal, kidney, liver, lung, multiple myeloma, myelodysplastic syndrome, non-Hodgkin's lymphoma, ovarian, pancreatic, prostate, stomach, and uterine. The non-cancer control cohort included age-matched, presumably healthy individuals and samples from individuals with non-cancer diseases to represent the intended use population more effectively.
Many cancers can be cured if detected early and treated effectively.i Yet cancer remains the second leading cause of death worldwide, accounting for approximately 1 in every 6 deathsii, with no recommended screening tests available for 70 percent of cancer diagnoses.
"Being able to screen patients for multiple cancers with a blood test, especially those that don't have a current screening option, is extremely exciting," said Anne Marie Lennon M.B.B.Ch., Ph.D. Director, Division of Gastroenterology and Hepatology and Professor of Medicine at Johns Hopkins Medicine. "Robust studies like this one are important in understanding the potential effectiveness of a multi-biomarker approach to MCED. This study shows encouraging signs that cancers, including early-stage cancers, can be detected, providing us the opportunity to improve patient outcomes by treating the disease when it is typically most responsive to therapy."
The study presented at ESMO builds on the insights and experience gained from earlier versions of the MCED test used in the DETECT-A study. DETECT-A, the first and only prospective, interventional study of 10,000 women to screen for multiple cancers, demonstrated the ability of an MCED test to more than double the number of screening-detected cancers compared to standard-of-care screening methods alone.iii
A larger case-control study is underway to further validate the results shared at ESMO and determine the final design of the MCED test. Exact Sciences will then begin recruiting patients for the FDA registrational Study Of All comeRs (SOAR) trial in MCED during 2023. The SOAR trial will be the largest prospective, interventional MCED trial ever conducted in the United States. Exact Sciences plans to leverage its leading presence in primary care and cancer screening to accelerate the availability of MCED and deliver this powerful innovation to patients in need.
About Exact Sciences Corp.
A leading provider of cancer screening and diagnostic tests, Exact Sciences relentlessly pursues smarter solutions providing the clarity to take life-changing action, earlier. Building on the success of Cologuard and Oncotype tests, Exact Sciences is investing in its product pipeline to support patients before and throughout their cancer diagnosis and treatment. Exact Sciences unites visionary collaborators to help advance the fight against cancer. For more information, please visit the company's website at exactsciences.com, follow Exact Sciences on Twitter @ExactSciences, or find Exact Sciences on Facebook.
Forward-Looking Statements
This news release contains forward-looking statements concerning our expectations, anticipations, intentions, beliefs or strategies regarding the future. These forward-looking statements are based on assumptions that we have made as of the date hereof and are subject to known and unknown risks and uncertainties that could cause actual results, conditions and events to differ materially from those anticipated. There can be no assurance as that future studies will successfully validate the data from the retrospective, case-control study discussed in this news release or that Exact Sciences will be able to successfully develop or market any multi-cancer early detection tests. Therefore, you should not place undue reliance on forward-looking statements. Risks and uncertainties that may affect our forward-looking statements are described in the Risk Factors sections of Exact Sciences' most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q, and in Exact Sciences' other reports filed with the Securities and Exchange Commission. Exact Sciences undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Media Contact:
Jack Hirschfield
jhirschfield@exactsciences.com
608-852-9877
Media (Canada, Europe, Asia, Latin America):
Federico Maiardi
+41 79-138-1326
Investors:
Megan Jones
+1 608-535-8815
i World Health Organization; February 2022 https://www.who.int/news-room/fact-sheets/detail/cancer
ii American Cancer Society, Global Facts and Figures 2022 https://www.cancer.org/research/cancer-facts-statistics/global.html
iii Science, April 2020 Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention